Abstract
Introduction: In cardiac amyloidosis (CA), immunoglobulin-derived light chains (AL) and transthyretin (TTR) are the most common amyloidogenic proteins infiltrating the heart. 1 Identification of the specific precursor protein leading to amyloid deposition is needed to establish the correct therapeutic approach. 2 In both AL- and TTR-related CA, an early and accurate diagnosis is critical to achieving the best treatment outcomes. TTR amyloid cardiomyopathy (ATTR-CM) is often misdiagnosed as other causes of heart failure (HF), including AL CA. 3 While almost all patients with AL amyloidosis have elevated serum free light-chain (sFLC) levels and abnormal free kappa:lambda (κ:λ) ratios, 4 patients with ATTR-CM can also have abnormal sFLC levels due to either an unrelated monoclonal gammopathy or relative κ-predominance in renal dysfunction. 2,5 Because ATTR-CM most often occurs in elderly adults and is commonly accompanied by renal comorbidity, we theorized that patients with ATTR-CM may have κ:λ ratios that approach, or exceed, the upper limit of the normal reference range (0.26-1.65 6). High light-chain ratios in these patients have the potential to increase the likelihood of misdiagnosis of a monoclonal plasma cell disorder and may lead to unnecessary referrals to hematologists and/or inappropriate treatments. To explore this theory, we evaluated κ:λ ratios in patients with biopsy-proven ATTR-CM who were enrolled in the Tafamidis in Transthyretin Cardiomyopathy Clinical Trial (ATTR-ACT). 7
Methods: In ATTR-ACT, a double-blind, placebo-controlled, randomized study, patients with biopsy-proven ATTR-CM, and without light-chain amyloidosis, received tafamidis or placebo for 30 months. In the current analysis, sFLC levels and κ:λ ratios were assessed in the intent-to-treat population (N=441), excluding patients with a prior diagnosis of monoclonal gammopathies (n=13; defined by MedDRA preferred terms: 'monoclonal gammopathy', 'plasma cell myeloma', 'plasma cell disorder', and 'hypergammaglobulinemia benign monoclonal'). Subgroup analyses were performed by estimated glomerular filtration rate (eGFR) category (≥60 vs ≥40 to <60 vs <40 mL/min/1.73 m 2). Findings were summarized using descriptive statistics (min/max, mean, median, and interquartile range [IQR]).
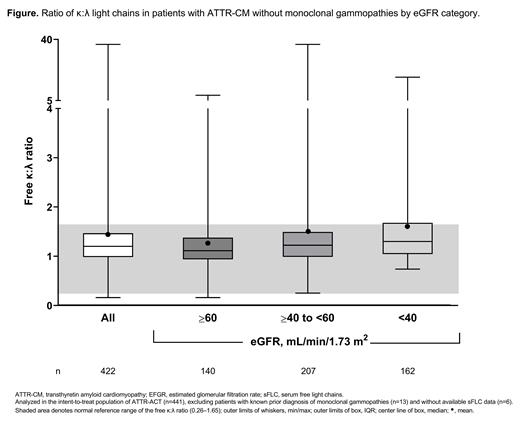
Results: In 422 patients with ATTR-CM and available sFLC data, and without a prior diagnosis of monoclonal gammopathies, the mean κ:λ ratio was 1.45 (median, 1.20 [IQR, 0.98, 1.47]) (Figure). The ratio increased with declining renal function: eGFR ≥60 mL/min/1.73 m 2, mean, 1.25 (median [IQR], 1.11 [0.94, 1.38]); ≥40 to <60 mL/min/1.73 m 2, 1.52 (1.22 [0.99, 1.49]); and <40 mL/min/1.73 m 2, 1.62 (1.30 [1.05, 1.68)].
Conclusions: In patients with biopsy-proven ATTR-CM without known monoclonal gammopathies who were enrolled in ATTR-ACT, the mean κ:λ ratio showed a κ-predominance, often exceeding the upper range of normal in patients with more advanced kidney dysfunction. The findings suggest that individuals with ATTR-CM can have higher sFLC levels than those normally seen in the general population, and such elevations do not necessarily indicate the presence of monoclonal gammopathy of undetermined significance or AL CA. In an era when most patients with ATTR-CM and without monoclonal gammopathies are diagnosed noninvasively using bone scintigraphy, age- and renal-function-based sFLC norms are critical to ensure appropriate use of diagnostic testing modalities.
References: 1. Maleszewski JJ. Cardiovasc Pathol. 2015;24:343-50. 2. Donnelly JP, et al. Cleve Clin J Med. 2017;84:12-26. 3. Witteles RM, et al. JACC Heart Fail. 2019;7:709-716. 4. Falk RH, et al. J Am Coll Cardiol. 2016;68:1323-41. 5. Geller HI, et al. Mayo Clin Proc. 2017;92:1800-5. 6. Katzmann JA, et al. Clin Chem. 2002;48:1437-44. 7. Maurer MS, et al. N Engl J Med. 2018;379:1007-16.
Hoffman: BMS, Celgene: Honoraria. Sultan: Pfizer: Current Employment, Current equity holder in publicly-traded company. Gundapaneni: Pfizer: Current Employment, Current equity holder in publicly-traded company. Witteles: Pfizer: Honoraria, Research Funding; Alnylam: Honoraria, Research Funding; Eidos: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal